eTARGET.
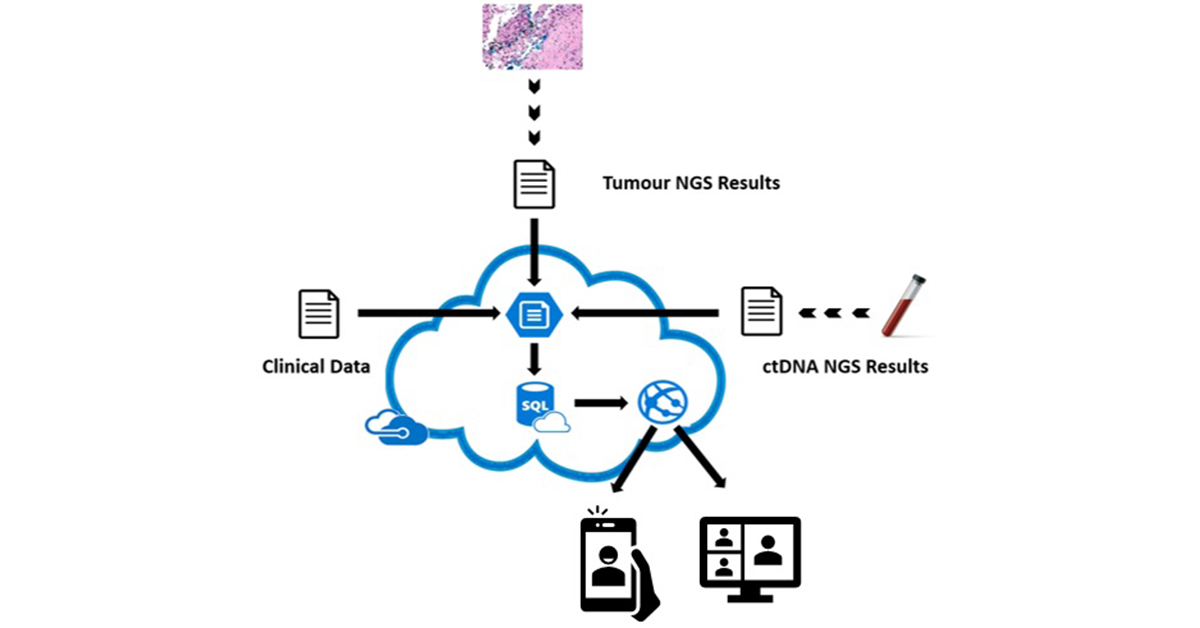
eTARGET – a paperless solution to overcome the challenge of integrating data from disparate sources in different organisations. It presents a single view of patient clinical and genomic NGS data to support decision making for Molecular Tumour Boards.
The eTARGET tool has revolutionised MTB. It facilitated continued patient review during the restrictions imposed by COVID-19 and has permitted smooth transition to national MTBs. Mutation frequency data is easily accessible to all trial investigators and the addition of the trial finder tool has enabled rapid national matching to clinical trials.
Key Features
CLINICAL
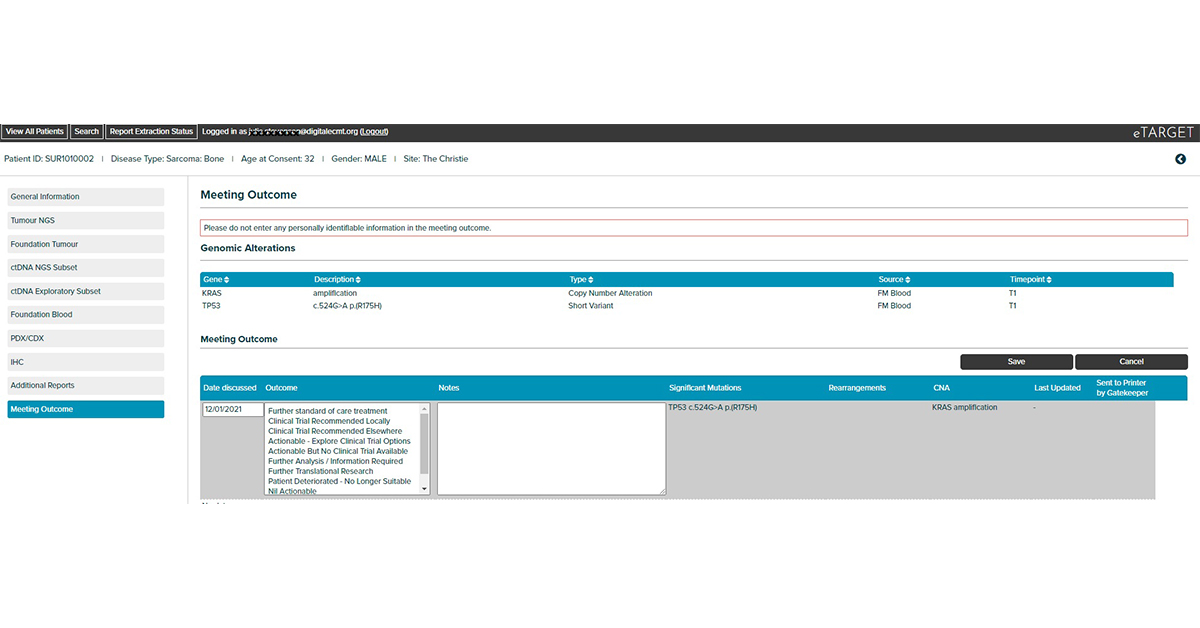
Integrates clinical and genomic data to support decision making by Molecular Tumour Boards. Developed by the Manchester Cancer Research Centre as part of the TARGET trial (Tumour chARacterisation to Guide Experimental Targeted therapy)
DATA
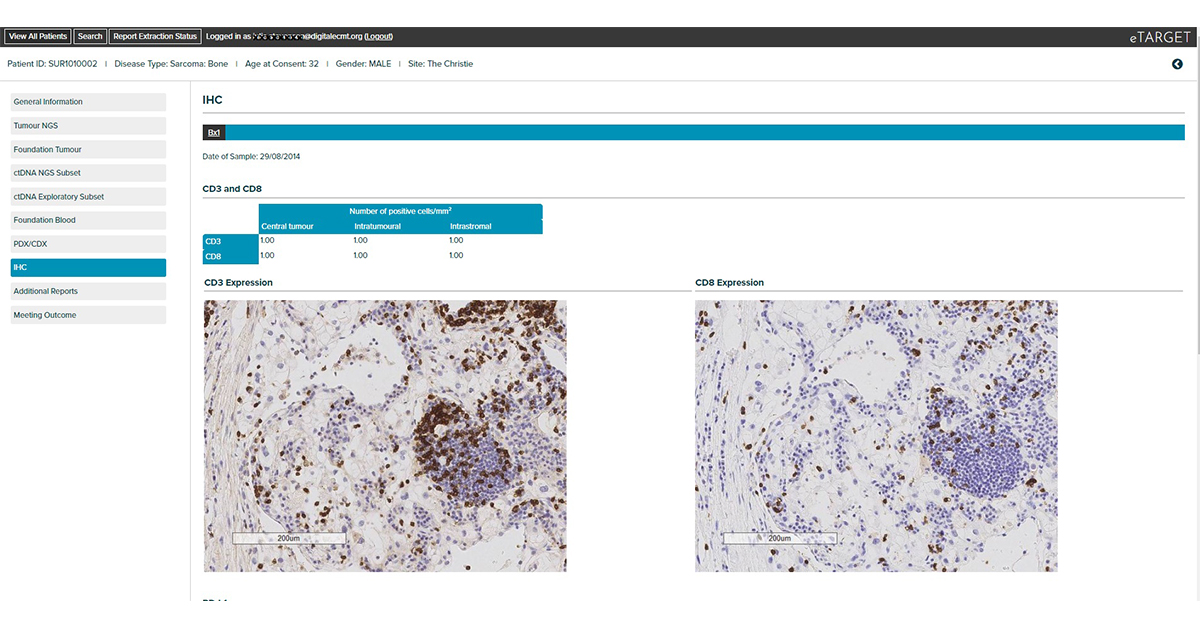
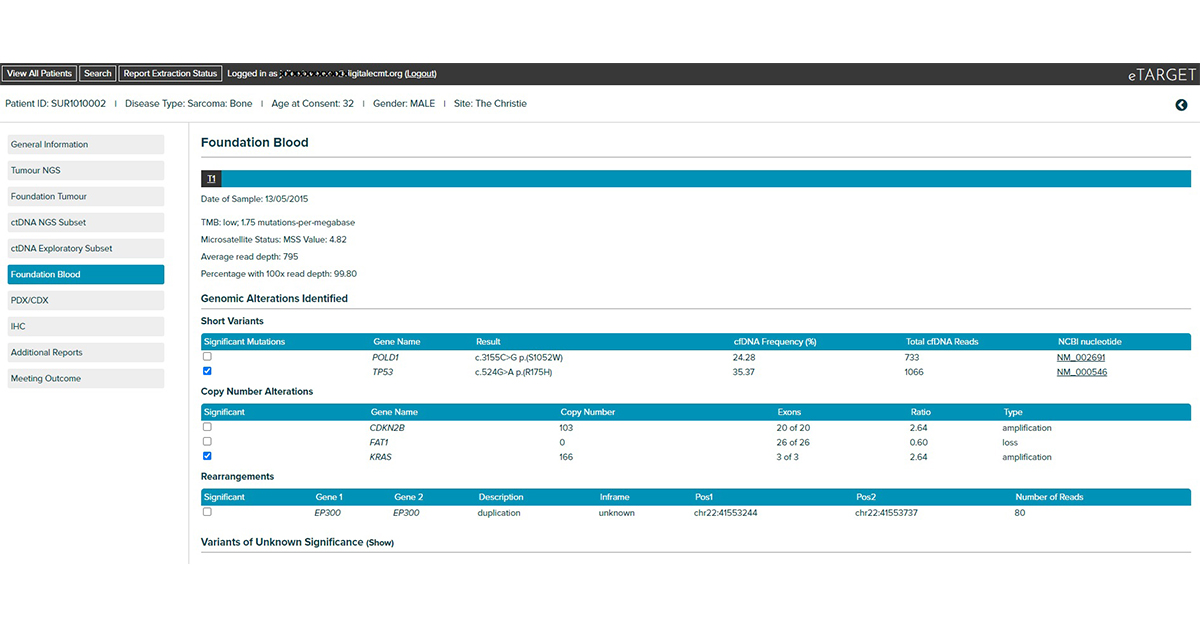
Integrates clinical, tumour NGS and circulating tumour DNA NGS data
TECHNICAL
eTARGET is a web solution deployed within Microsoft Azure.
How can I use eTARGET at my centre?
eTARGET requires basic technical infrastructure, comprising a web server, a database and secure storage. This can be deployed on any major cloud provider (e.g. Azure, AWS) or on site (Academic Institution or NHS Trust).
Once installed, it will require basic technical monitoring on the infrastructure side. On the application side, it will require i) user management and ii) file uploading. Further details are available at the links below.
Access the product
Front-end and web services (MIT Licence)
Data Ingest Process (GLP 3.0 Licence)
QUICK LINKS
Home
About
Disclaimer: The digital health products created through UpSMART are for research use only as regulatory approval has not yet been sought. Please contact us if you wish to use any of the UpSMART products in a research study.
CONTACT
Digital Cancer Research team,
Cancer Research UK Manchester Institute
The University of Manchester
Wilmslow Road
Manchester
M20 4BX