Proact 2.0
Patient Reported Opinions About Clinical Tolerability – PROACT 2.0 is an open source patient application for secure, direct communication between clinical trial participants and their medical team.
PROACT 2.0 was developed by the team at Fondazione IRCCS Istituto Nazionale dei Tumori di Milano, Milan Italy, in collaboration with UpSMART Work Package 2. It is accessible via a mobile phone app or website and compatible with both iOS and Android operating systems.
Latest news:
On 26th October 2023 the 8th edition of “Forum Sanità” was held in Rome: the aim of the award, promoted by the Digital360 Group, was to highlight innovative projects in the broader field of Health, which are based on cutting-edge technologies, introducing innovative elements of process, decision support and hospital-territory integration.
In the Citizen Journey area, dedicated to patients-centered projects, the project of PROACT 2.0 was awarded by the scientific commission with the following reason:
“… because it shortens the distance between the oncological patient and the medical team, not only in the sphere of treatment but also in that of clinical research, which sees the patient subjected to various stresses in terms of collecting data and information, as well as having to fulfil bureaucratic and administrative needs… ”
In an era of more patient-centered care pathway and rising complexity, improving effective communication between patients and their medical team and collecting PROs is more important than ever. It’s exciting to work in a multidisciplinary team of software developers and clinicians in developing PROACT 2.0, a platform which helps researchers to reach this goal.
Key Features
CLINICAL
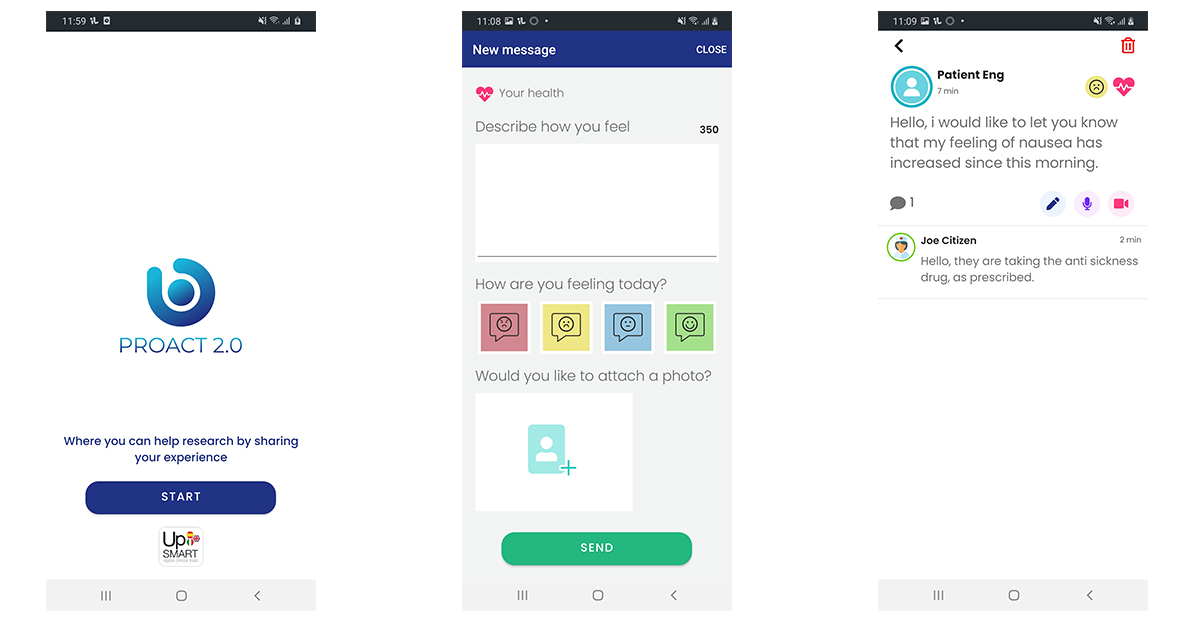
PROACT 2.0 enables patients to communicate with their medical team in ‘real time’
Text, audio and video messaging functionality
Easy access via mobile app or web browser
Gives patients the opportunity to engage in their medical care
All communication secure and confidential
Enables medical teams to communicate with their patients directly and securely
Text, audio and video messaging functionality
Functionality to analyse patient messages ‘real time’
DATA
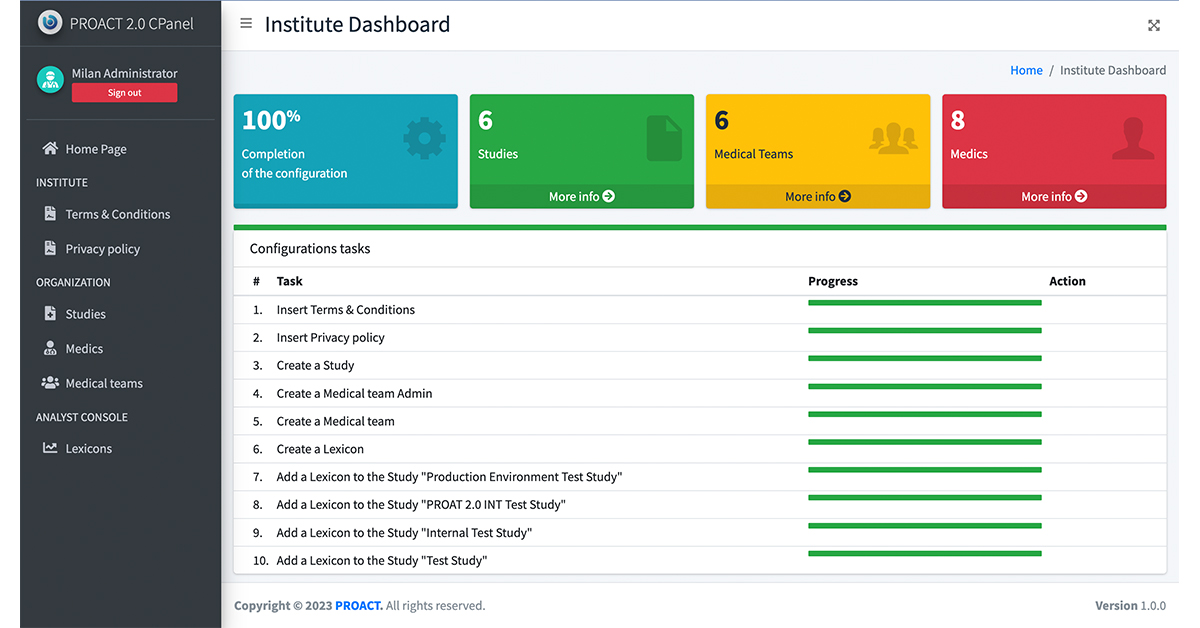
Study Set up – Control Panel
Enables system administrator to set up studies with specific functionality required
Functionality to build in surveys for patient feedback
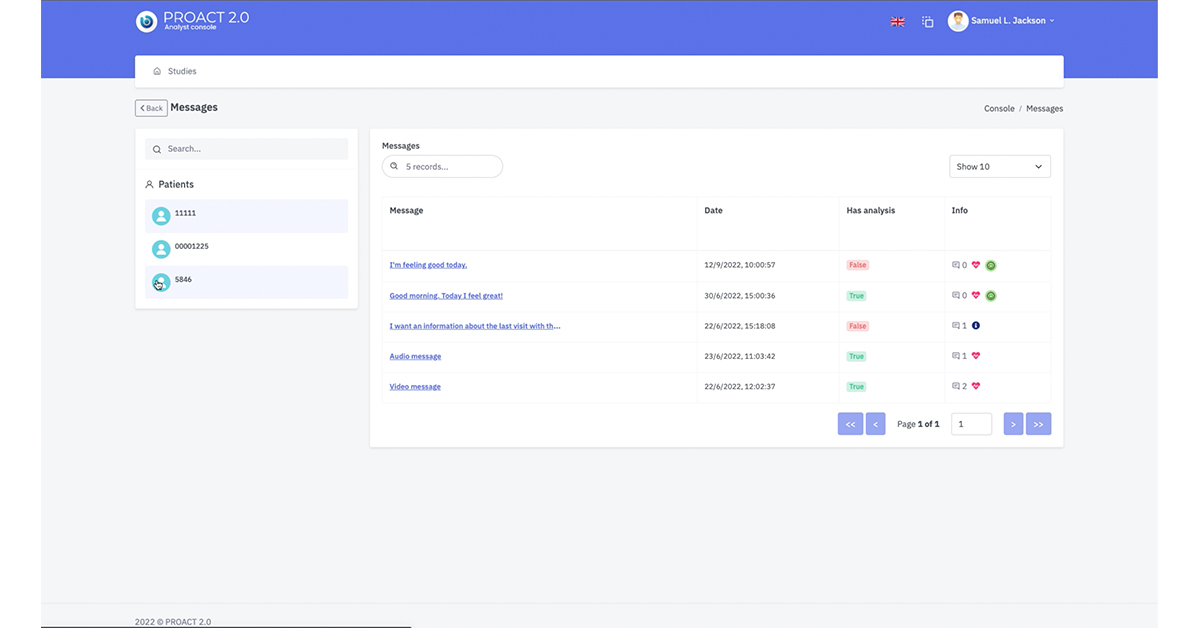
Analyst Console
Built in functionality allowing medical team to analyse patient messages
Functionality for study specific lexicons to be uploaded for message analysis
Allows multiple analysis by multiple team members
Functionality to extract data for further message analysis
Surveys
Data Collection – Patient Feedback
Built in functionality allowing medical team to create a trial specific survey
7 different types of question types available to add to a survey: Open answer, Single choice, Multiple choice, Boolean, Rating, Mood
Validation of survey prior to patient distribution to ensure quality and integrity
Functionality to assign the survey to patients within the trial
on a daily, weekly or monthly basis
Patient alerts for new surveys to be completed
Medic interface allows access to completed patient surveys
Data analysis
Includes dashboard functionality to allow the medical team to analyse information collected in the surveys
Allows statistical information and aggregation of data for analysis
Hovering over the visualisation presents a tooltip with further information
TECHNICAL
PROACT 2.0 is a Mobile Application for iOS and Android created using Microsoft Xamarin and .NET technologies
PROACT 2.0 Backend is created using .NET Core technologies and Microsoft Azure
PROACT 2.0 Analyst Console is created using React JS accessible through Web Browser
PROACT 2.0 Control Panel is created using .NET MVC Framework and is accessible using Web Browser.
Access the product
PROACT 2.0 Services Documentation
User Manuals
PROACT 2.0 Services Installation Guides
QUICK LINKS
Home
About
Disclaimer: The digital health products created through UpSMART are for research use only as regulatory approval has not yet been sought. Please contact us if you wish to use any of the UpSMART products in a research study.
CONTACT
Digital Cancer Research team,
Cancer Research UK Manchester Institute
The University of Manchester
Wilmslow Road
Manchester
M20 4BX